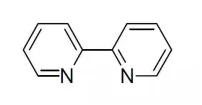
【English name】2,2-Bipyridine
【Molecular formula】 C10H8N2
【Molecular Weight】 156.2
【CAS】366-18-7
【Abbreviations and aliases】α,α’-Dipyridyl,2,2-Bipyridine
【structural formula】
【Physical properties】White or light red crystalline powder, bp272~273 oC. Soluble in alcohol, ether, benzene, chloroform, and petroleum ether; soluble in water, its aqueous solution will appear red when it meets ferrous salt.
【Precautions】 This reagent is flammable and will produce toxic nitrogen oxide fumes when burned. Store 02 reactions in a fume hood.
2,2-Bipyridine is a very important organic ligand in organic chemistry, and because of its aromaticity, it can also undergo a series of reduction reactions, oxidation reactions, and C−H bond activation reactions.
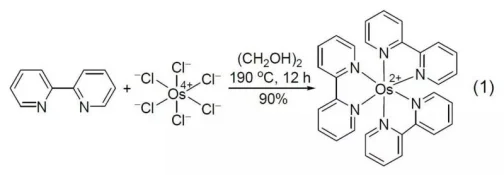
2,2-Bipyridine reacts with osmium hexachloride overnight at high temperature to form the corresponding complex (Formula 1).
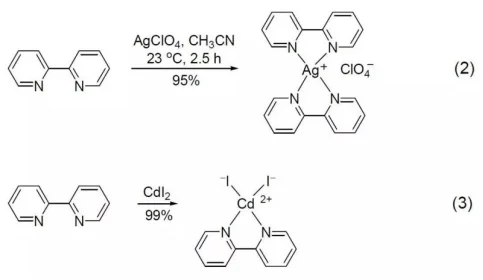
The reaction of 2,2-Bipyridine with silver perchlorate (I) at room temperature can generate the corresponding complex (Formula 2), and the reaction with cadmium diiodide (II) can obtain pyridine and iodine coordination in cadmium The above product (Equation 3)
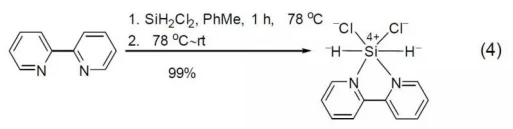
2,2'-bipyridine can coordinate not only with metal but also with non-metal atom silicon. As shown in Formula 4:2,2-Bipyridine and dihydrosilane dichloride can form corresponding complexes in toluene solvent.
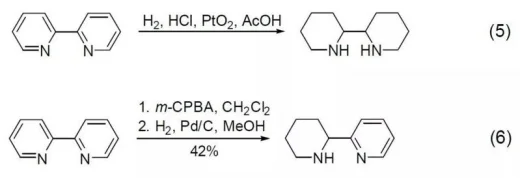
In addition to the outstanding coordination ability, because pyridine itself is an aromatic system, it also has the typical properties of aromatic compounds. Using hydrogen to reduce 2,2-Bipyridine in platinum dioxide hydrochloric acid acetic acid solution, the product with both pyridine rings completely reduced can be obtained (Formula 5). If m-CPBA is used to oxidize first, and then Pd/C is used to catalyze the hydrogenation reduction, the product with only one pyridine ring reduced can be obtained (Formula 6).
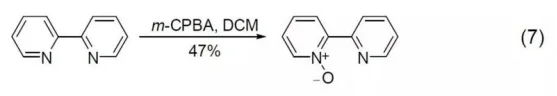
2,2-Bipyridine can be oxidized to 2,2-Bipyridine-1-nitroxide using m-CPBA (Equation7).
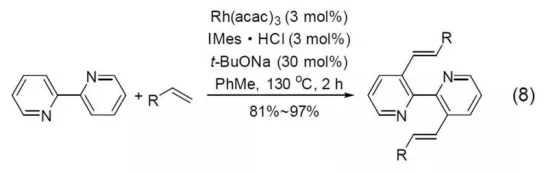
Under the catalysis of rhodium complexes, 2,2-Bipyridine and olefin compounds undergo double carbon-hydrogen activation reactions at the 3-position and 3’-position (Formula 8).
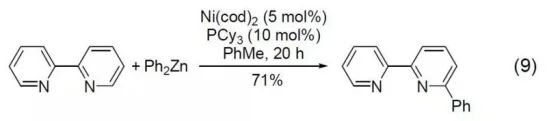
The reaction of 2,2-Bipyridine and diphenylzinc reagent under the catalysis of nickel complex can generate 6-phenyl-2,2-Bipyridine (Formula 9).